This lecture can be viewed on YouTube site: Life Scinence Lectures for you
https://youtu.be/(in preparation)
1. Overview
This lecture explains the pathway by which 24-nt siRNAs mediate de novo introduction of 5-methylcytosine (5mC) at CH, CHG, and CHH sites in plants. It also covers the H3K9me2 histone modification induced by 5mC and the maintenance DNA-methylation.
In seed plants, RNA-directed DNA methylation (RdDM) suppresses the expression of transposons, repetitive sequences, and viruses by blocking the initiation of their transcription.
Key Words: transposon, H3K9me2, siRNA, Pol II, Pol IV, Pol V, 5mC, double-stranded RNA (dsRNA), non-canonical RdDM, DRM2, MET1, CMT3, CMT2
2. Stable Gene Silencing by DNA Methylation and Histone Modifications

In eukaryotes, DNA cytosine methylation (5-methylcytosine, 5mC) and histone modifications act cooperatively to achieve long-term, stable repression of gene expression. These epigenetic marks promote chromatin compaction, establishing a persistent heterochromatin structure.
Genes located within heterochromatic regions become inaccessible to RNA polymerase, thereby preventing transcription initiation. Representative repressive histone modifications include H3K9me2—di-methylation of the ninth lysine (K) residue of histone H3—in plants, and H3K9me3 in animals.
3. Active Transposable Elements in Angiosperms
n the human and mouse genomes, transposable elements that remain capable of mobilization are extremely limited (e.g., LINE-1 and certain members of the Alu family of SINEs). By contrast, angiosperm (plants that have flowers and seeds) genomes contain numerous transposons that are still highly active today : Examples of DNA (cut-and-paste) transposons include Ac/Ds, MuDR, Spm/En, and Tam3, while examples of RNA (copy-and-paste/retrotransposons) transposons include Tos17, Tnt1/Tto1, and Onsen.
To suppress the movement of these transposons, angiosperms have evolved not only RNA interference (RNAi) but also a mechanism known as RNA-directed DNA methylation (RdDM), which closely resembles the RNAi pathway. Both pathways produce short double-stranded RNAs and utilize Argonaute, Dicer, and RNA-dependent RNA polymerase (RDRP) proteins.
Whereas RNAi acts post-transcriptionally on mRNA to inhibit translation, RdDM introduces de novo methylation of cytosine residues (5-methylcytosine), thereby silencing transposons at the transcriptional level. RdDM requires two plant-specific RNA polymerases—Pol IV and Pol V—to transcribe target loci, making this pathway unique to plants.
4. Examples of RNA-Directed DNA Methylation (RdDM) Acting on Foreign Genes
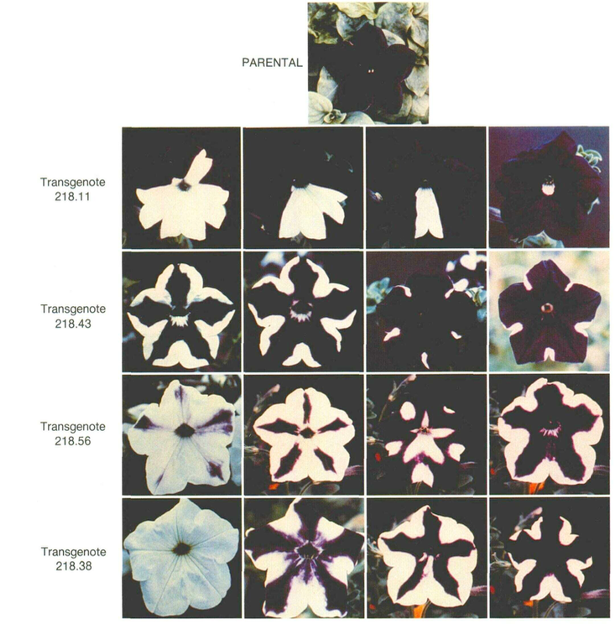
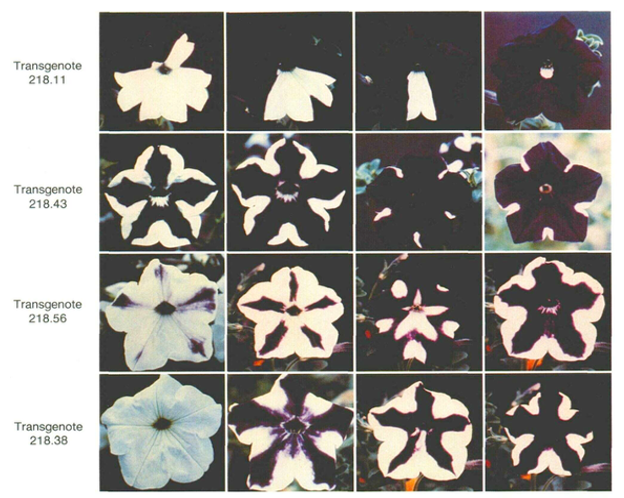
The targets of RdDM-mediated transcriptional repression are not limited to transposons or endogenous repetitive sequences. Transgenes—artificial genes introduced by genetic engineering—can also undergo RdDM-dependent silencing, depending on their insertion site and copy number within the genome.
A case that led to the discovery of the RdDM pathway in plants involved the chalcone synthase (CHS) gene, which encodes the rate-limiting enzyme for anthocyanin (purple pigment) biosynthesis. When an extra copy of CHS was introduced and expressed in the genome, flowers displayed strikingly variegated phenotypes: purple petals with white patches, or entirely white petals.
In the white regions, mRNAs derived from both the transgene and the endogenous CHS gene were markedly reduced, showing that transcription of both sequences was silenced. This phenomenon was termed co-suppression.
Subsequent studies revealed heavy accumulation of 5-methylcytosine (5mC) in both the transgene and the endogenous gene within co-suppressed cells, and demonstrated that 24-nt small interfering RNAs (siRNAs) play a crucial role in this DNA methylation.
This lecture explains how angiosperms and gymnosperms employ RdDM to achieve stable transcriptional silencing of transposons, repetitive elements, viruses, and even transgenes.
5. Translational repression by RNA interference involving 21-nt siRNA
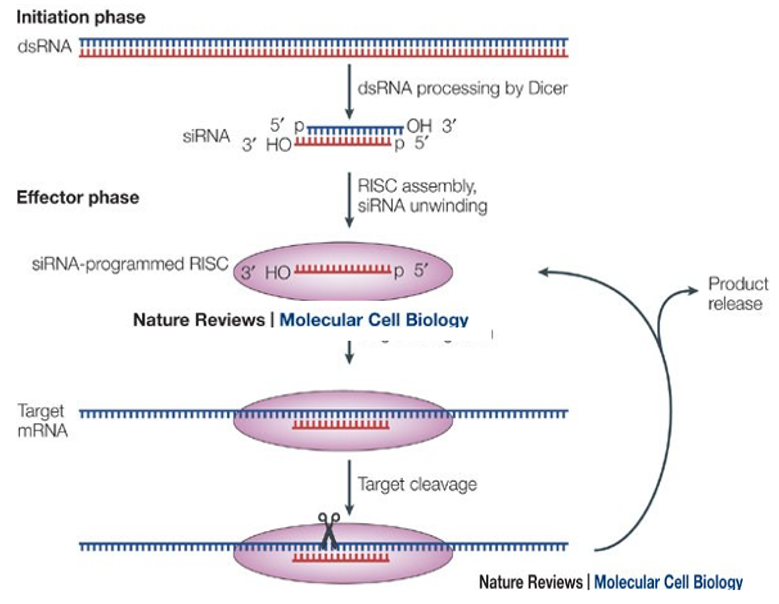
RNA-directed DNA methylation (RdDM) is considered a relatively recent mechanism that emerged during the evolution of land plants, derived from the more ancient RNA interference (RNAi) pathway. First, let’s briefly review RNAi (For a detailed explanation of RNAi, please refer to a separate lecture).
In the RNAi pathway, mRNAs derived from viruses or transposons are converted into double-stranded RNA (dsRNA) by RNA-dependent RNA polymerase (RDRP). These dsRNAs are then cleaved by Dicer into double-stranded fragments of 21 nucleotides. One strand of each fragment is incorporated into an Argonaute protein, and together with several other proteins forms an active RNA-Induced Silencing Complex (RISC). Within RISC, the siRNA base-pairs with complementary sequences in specific target mRNAs.
6. Cleavage or degradation of target mRNA by RISC binding
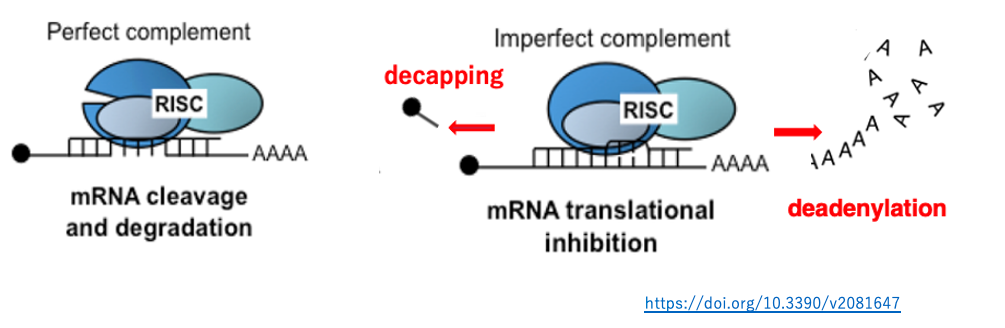
When the complementarity between siRNA and its target mRNA is very high, the mRNA is cleaved (left figure). If the siRNA–mRNA pairing contains regions of partial mismatch, cleavage does not occur; instead, translation inhibition takes place through mechanisms such as the promotion of mRNA degradation (right figure). Such RNAi-mediated translational repression is observed in a wide range of eukaryotes, suggesting that this mechanism was acquired early in eukaryotic evolution.
RNA interference (RNAi) is thought to act as a defense mechanism that prevents the activation of transposons and viruses that could harm the host by repressing their translation.
Subsequently, an RNAi pathway involving microRNAs (miRNAs) evolved as a mechanism to regulate the translation of endogenous genes.
7. Pol IV and Pol V Evolved from Pol II in Angiosperms
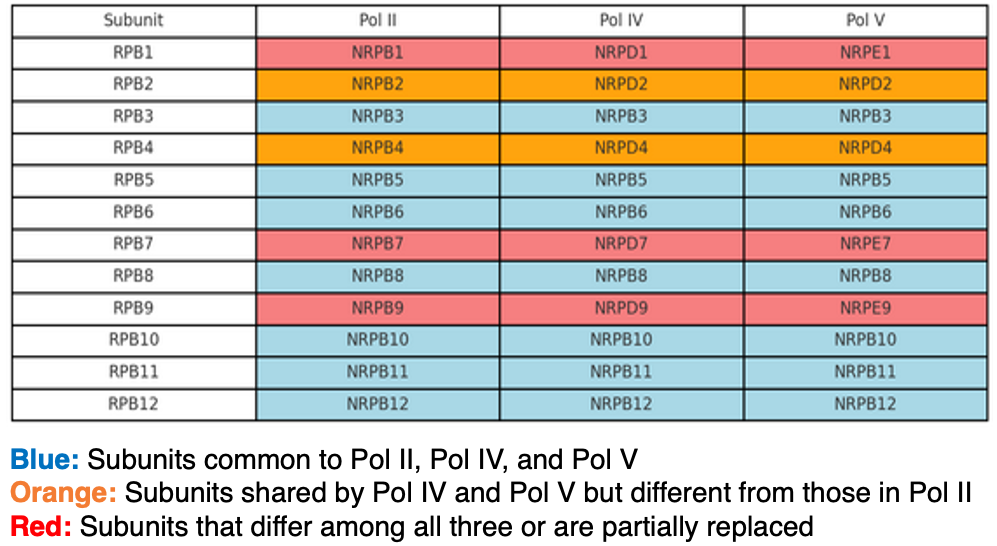
In eukaryotes, RNA polymerase II (Pol II) is the conventional RNA polymerase that transcribes protein-coding genes, and it is composed of 12 subunits. Pol IV and Pol V—found only in seed plants—are also made up of 12 subunits.
As shown in this figure, the three polymerases share seven subunits (light blue), while three subunits are unique to each (red). In addition, Pol IV and Pol V share two subunits with each other but not with Pol II (orange). Because of these shared subunits, Pol IV and Pol V are thought to have arisen from Pol II during the evolution of seed plants.
Pol IV and Pol V are key components of the RNA-directed DNA methylation (RdDM) pathway, which is therefore found only in seed plants. Accordingly, this pathway is absent in mosses and ferns.
8. Cytosine Methylation in Seed Plants
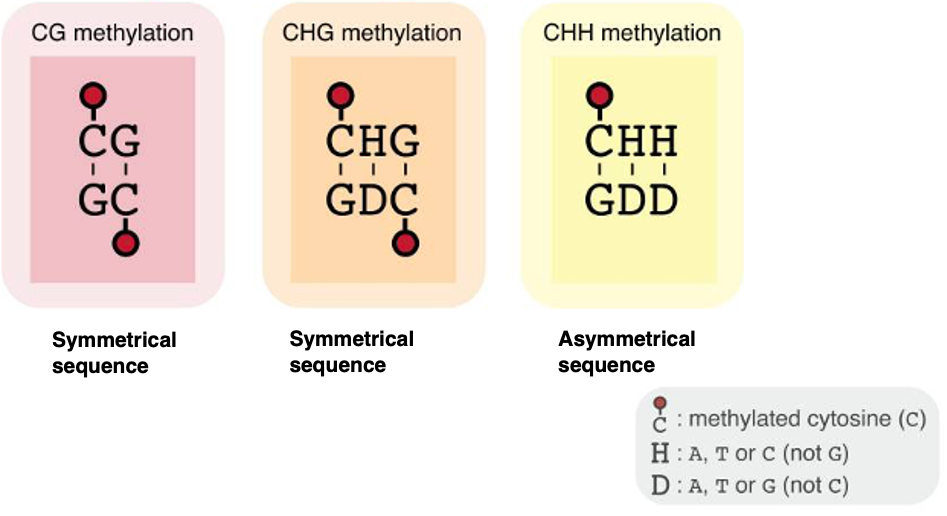
In animal genomes, cytosine methylation typically occurs at cytosines in the 5′-CpG-3′ sequence context (5-methylcytosine, 5mC). In contrast, the genomes of seed plants can also exhibit cytosine methylation in CHG and CHH contexts, where H represents A, T, or C but not G.
While CG and CHG sites are symmetrical when considering double-stranded DNA, CHH sites are asymmetric.
When a DNA molecule containing methylated cytosines on both strands is replicated, the newly synthesized daughter strand initially lacks methylation (a hemimethylated state).
At CG and CHG sites, cytosines at positions complementary to parental 5mC can be methylated by maintenance methyltransferases.
However, because the complementary cytosine is absent at parental mCHH sites, maintenance methyltransferases cannot methylate the daughter strand using symmetry as a guide.
As a result, CHH methylation is more easily lost during DNA replication compared with CG or CHG methylation.De novo methylation is therefore always required to establish and maintain CHH methylation.
9. Comparison of Methylation Frequencies by Sequence Context in Angiosperms
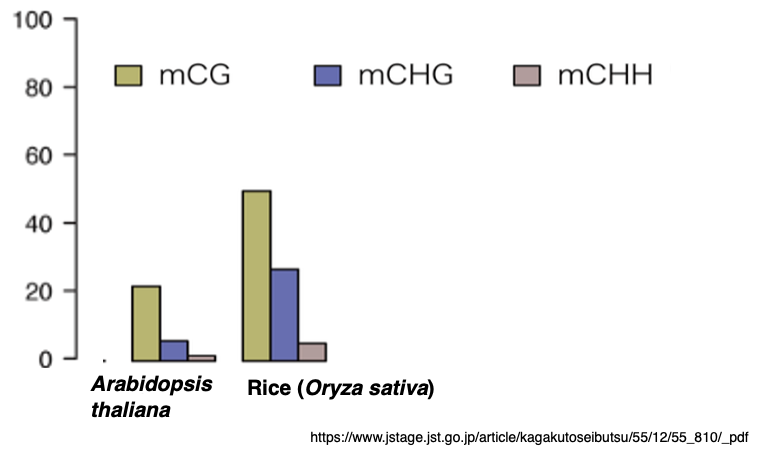
This figure shows the frequencies of CG, CHG, and CHH methylation in Arabidopsis thaliana and rice.
In both species, CG methylation occurs at the highest frequency, whereas CHH methylation is the least frequent.
When 5-methylcytosine (5mC) is present at high levels within genes or in their surrounding regions, transcription initiation is repressed.
However, even when 5mC is present within a DNA sequence, RNA polymerase that has already initiated transcription does not slow down or stall. Once transcription begins, the polymerase can proceed along the DNA just as efficiently as it would on an unmethylated template.
10. Mechanisms of Transcription Initiation Repression by DNA Methylation
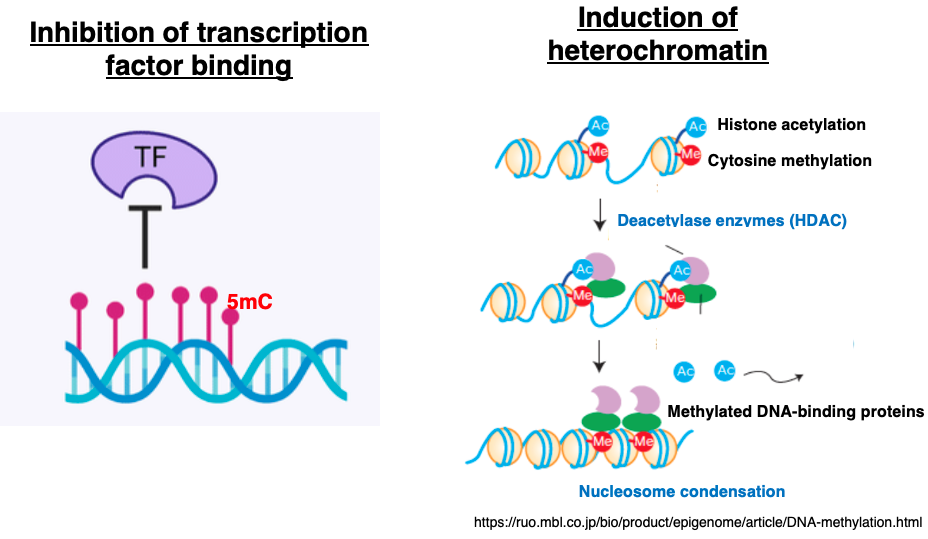
Two primary mechanisms are thought to explain how 5-methylcytosine (5mC) suppresses transcription initiation:
- Interference with Transcription Factor (TF) Binding
When promoter or enhancer motifs that serve as transcription factor binding sites become methylated, the binding affinity of the relevant TFs decreases. This reduced binding lowers the frequency of transcription initiation. - Recruitment of Chromatin-Modifying Enzymes via 5mC Marks
5mC acts as a molecular marker that recruits histone deacetylases and histone methyltransferases.
These enzymes introduce repressive histone modifications, such as H3K9me2, leading to the conversion of euchromatin into heterochromatin.
As a result, RNA polymerase II and the transcription initiation complex can no longer access the DNA.
Together, these two mechanisms are believed to inhibit the start of transcription.
11. Simplified Pathway of RNA-Dependent DNA Methylation (RdDM)
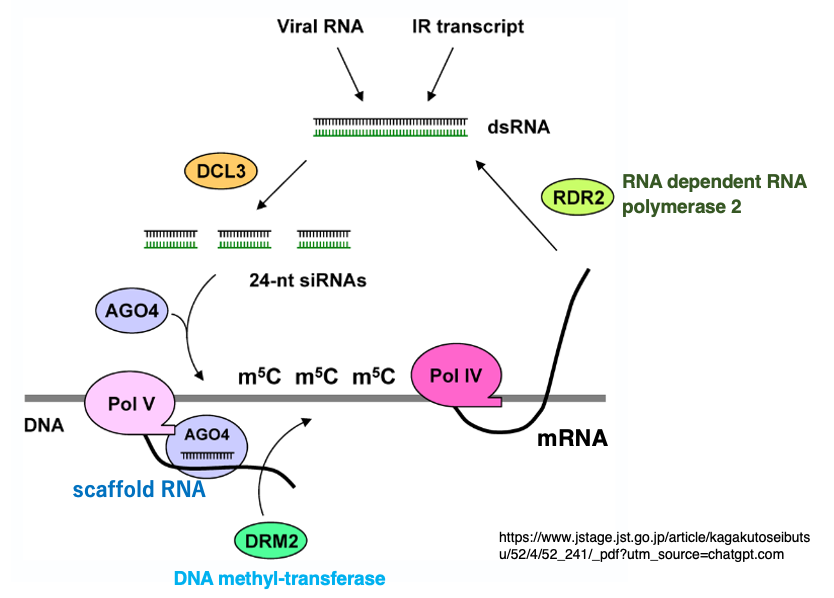
DNA sequences encoding transposons or repetitive elements often contain low levels of pre-existing 5-methylcytosine (5mC). Such loci are transcribed not by the usual RNA polymerase II (Pol II) but by RNA polymerase IV (Pol IV), producing RNA transcripts.
These transcripts are converted into double-stranded RNA (dsRNA) by RNA-dependent RNA polymerase 2 (RDR2).
The dsRNA is then diced into 24-nucleotide small interfering RNAs (siRNAs) by Dicer-like 3 (DCL3), and one strand of each siRNA is incorporated into Argonaute RISC Component 4 (AGO4).
Meanwhile, RNA polymerase V (Pol V) transcribes nearly the same genomic regions as Pol IV, generating a scaffold RNA.
AGO4, carrying the siRNA, binds to this scaffold RNA through base-pair complementarity between the siRNA and the scaffold transcript.
This interaction recruits Domains Rearranged Methyltransferase 2 (DRM2), a de novo DNA methyltransferase.
DRM2 introduces cytosine methylation (5mC) without regard to CG, CHG, or CHH context within roughly ±200–500 bp of the siRNA-targeted region.This summarizes the de novo DNA methylation pathway mediated by 24-nt siRNAs. Notably, siRNAs do not directly base-pair with the target DNA itself. Instead, they guide AGO4 to the scaffold RNA, and this complex recruits DRM2 to methylate the surrounding DNA.
12. Detailed Pathway of RNA-Dependent DNA Methylation (RdDM)
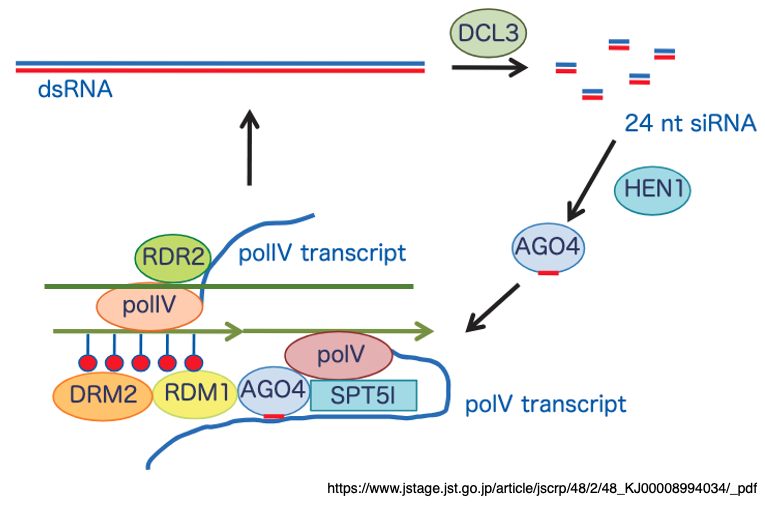
This figure illustrates a more detailed view of the RdDM pathway. Transposons and repetitive DNA sequences that contain 5-methylcytosine (5mC) are sometimes transcribed not by RNA polymerase II (Pol II) but by RNA polymerase IV (Pol IV).
The Pol IV-derived transcripts are converted into double-stranded RNA (dsRNA) by RNA-dependent RNA polymerase 2 (RDR2).
This dsRNA is then cleaved by Dicer-like 3 (DCL3) into 24-nucleotide small interfering RNAs (siRNAs).
The 3′ ends of these siRNAs are stabilized through 2′-O-methylation catalyzed by HEN1.One strand of each siRNA is incorporated into Argonaute 4 (AGO4) and binds, via base-pair complementarity, to scaffold RNAs that are transcribed by RNA polymerase V (Pol V).
Pol V transcription is supported by SPT5I (Suppressor of Ty insertion 5-like), a Pol V-specific transcription elongation factor.
RDM1 (RNA-Directed DNA Methylation 1) is a small (~200 aa) protein that assists in recruiting the de novo DNA methyltransferase Domains Rearranged Methyltransferase 2 (DRM2) to the AGO4–siRNA complex.
DRM2 catalyzes cytosine methylation without regard to CG, CHG, or CHH context, within approximately ±200–500 bp of the siRNA-targeted region.
13. Maintenance DNA Methyltransferase–Mediated Hemimethylated CG Sites
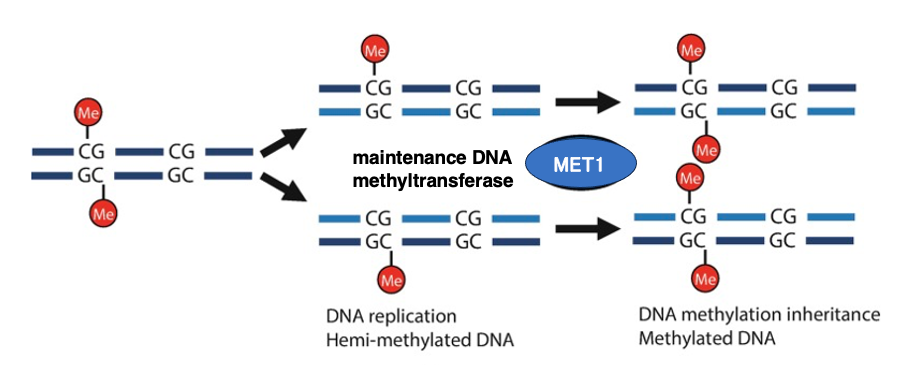
When DNA containing 5-methylcytosine (5mC) is replicated, the parental strand retains its methylation, while the newly synthesized (daughter) strand initially lacks methyl groups, creating a hemimethylated state.
At CG sites, the maintenance DNA methyltransferase MET1 acts on the cytosine in the daughter strand opposite the 5mCG on the parental strand, introducing a methyl group. The efficiency of this MET1-mediated methylation of the nascent strand is considered relatively high.
14. Methylation of Hemimethylated Strands: CHG and CHH Contexts

For CHG sequences, the maintenance DNA methyltransferase CMT3 recognizes the daughter-strand cytosine opposite a 5mCHG on the parental strand and introduces methylation.
However, this enzyme shows little activity unless the associated histones carry the H3K9me2 modification.
Because CHH sites lack symmetry, there is no maintenance enzyme capable of methylating the unmodified cytosine on the newly synthesized strand.
15. Methylation of the Nascent Strand Mediated by H3K9me2 Histone Modification
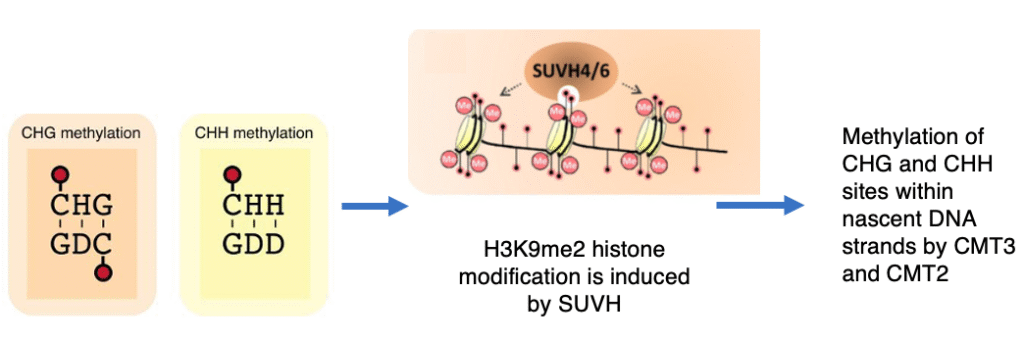
Histone methyltransferases such as SUVH4, SUVH5, and SUVH6 recognize 5-methylcytosine (5mC) and bind to the DNA, adding the H3K9me2 modification to histone H3.
This histone mark activates the maintenance methyltransferase CMT3, which methylates hemimethylated CHG sites on the newly synthesized strand.
In addition, the presence of H3K9me2 recruits CMT2, which introduces methylation at CHH sites on the nascent strand.
16. Mechanism of de novo 5mC Introduction and Maintenance via RdDM
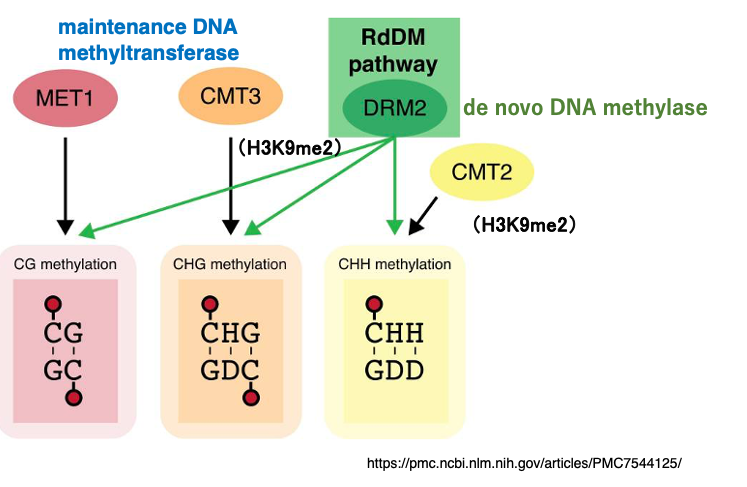
For CG sequences, even in the absence of H3K9me2 modification, the maintenance-type DNA methyltransferase MET1 methylates the cytosines on the daughter strand at the corresponding positions.
In contrast, for CHG and CHH sequences, the histone methyltransferase SUVH introduces H3K9me2 modification, which recruits the DNA methyltransferases CMT3 and CMT2 to methylate CHG and CHH sites on the nascent strand.
Meanwhile, the de novo methyltransferase DRM2, bound to scaffold RNA, introduces 5mC into cytosines—without distinction among CG, CHG, or CHH—within approximately ±200–500 bp of the target region.
17. Pol IV and Pol V-independent RdDM targeting newly introduced transgenes
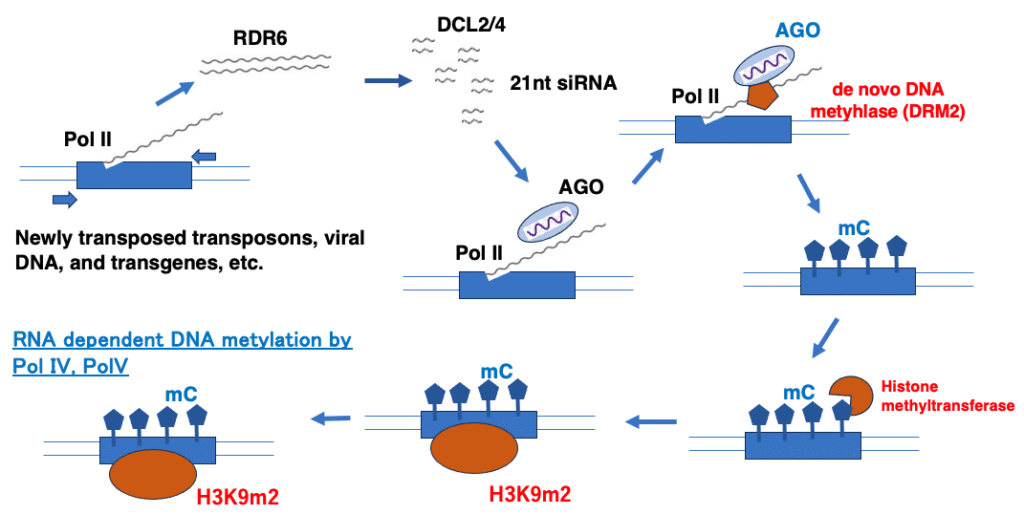
Pol IV/Pol V-Independent RdDM Acting on New Transgenes and Other Sequences
Transposons and repetitive sequences that are transcribed by Pol IV or Pol V often already carry low levels of 5-methylcytosine (5mC) and H3K9me2 modifications. These epigenetic marks serve as cues to recruit Pol IV and Pol V.
However, RNA-directed DNA methylation (RdDM) can also act on newly mobilized transposons, transgenes introduced by genetic engineering, or viral DNA inserted into the genome during infection—even when such marks are absent.
In this case, the pathway proceeds as follows:
- Pol II transcribes the gene.
- The resulting mRNA is converted into double-stranded RNA by RDR6.
- DCL2/4 processes this dsRNA into 24-nt siRNAs.
- A Pol II (or Pol V) transcript serves as the scaffold RNA to which the AGO–siRNA complex binds.
- DRM2 is recruited, triggering de novo DNA methylation across sequence contexts, independent of CG/CHG/CHH specificity.
This Pol IV/Pol V-independent route is known as the non-canonical RdDM pathway. Once DNA methylation is established, histone methyltransferases are recruited, depositing H3K9me2 marks.
Through this non-canonical RdDM, introduction of 5mC and H3K9me2 into the gene renders it a target for the conventional Pol IV/Pol V-dependent RdDM pathway.
18. Summary of Canonical and Non-Canonical RdDM Pathways
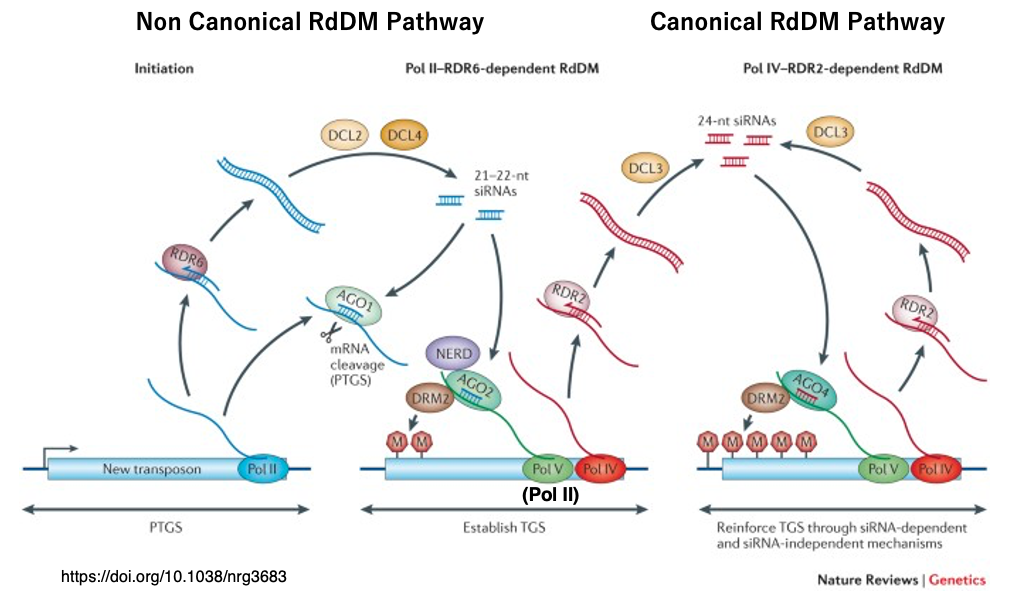
When a new transposon is transcribed by Pol II, part of the transcript is converted into double-stranded RNA (dsRNA) by RDR6, and DCL2/DCL4 process it into 21–22 nt siRNAs.
These siRNAs induce post-transcriptional gene silencing (PTGS) through AGO1, but a portion of the dsRNA is also loaded into AGO2.
Activated AGO2 binds to scaffold RNA—a transcript of Pol V (or Pol II)—and recruits the de novo DNA methyltransferase DRM2, which introduces 5-methylcytosine (5mC) into the surrounding DNA sequences.
This initial DNA methylation recruits Pol IV/Pol V, thereby activating the canonical RdDM pathway.
In the canonical RdDM pathway, 24-nt siRNAs are produced.
19. Short- and Long-Distance Signaling of siRNA
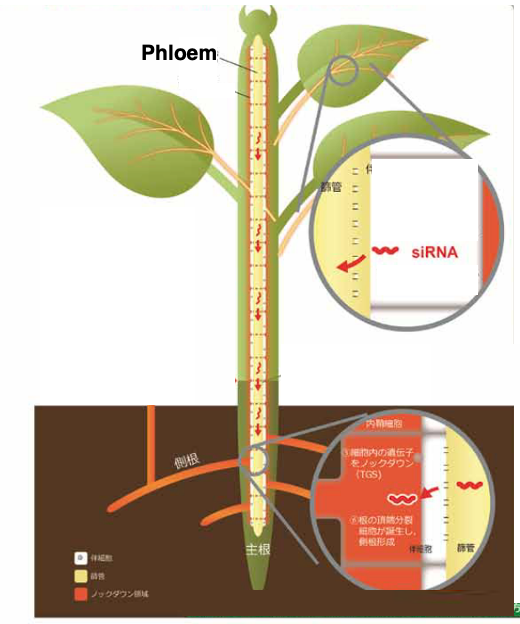
It has been experimentally demonstrated that 24-nt siRNAs can move long distances through the phloem and induce ectopic canonical RdDM in recipient tissues.
This phenomenon is involved in various plant physiological processes such as grafting, antiviral defense, and intergenerational information transfer.
Such systemic spreading of RdDM transmits stress-response information to the germline, contributing to enhanced resistance in the next generation.In addition, siRNAs are also known to move between neighboring cells through plasmodesmata.
コメント